Published: June 9, 2023
Vessela Mavrodieva (USDA-APHIS-PPQ-S&T, PPCDL)
Hello NPDN colleagues,
Vessela Mavrodieva from USDA’s Plant Pathogen Confirmatory Diagnostic Lab (PPCDL) here. I want to update you on some exciting changes coming to the PPQ lab accreditation and certification program. Many of you have participated in the National Plant Protection Laboratory Accreditation Program (NPPLAP) and have been certified for regulatory diagnostics of P. ramorum, citrus greening, and plum pox virus for years. PPQ values this partnership, and we believe that proficiency testing is an essential tool to ensure we get diagnostic results right and help expand nation-wide laboratory capacity for regulatory plant pathogens testing. On behalf of PPQ and NPPLAP, thank you for your past efforts and support.
While the NPPLAP has enjoyed nearly 20 years of success, it does have limitations that make it difficult to effectively expand the program. PPQ’s need for competent molecular diagnosticians continues to expand, outpacing our resources. So, we set about redesigning the program to provide certifications for commonly used molecular techniques applicable for testing many plant pathogens, rather than pathogen-specific protocols. This new, flexible-scope program is named the Plant Pathogen Diagnostic Certification Program (PPDCP). 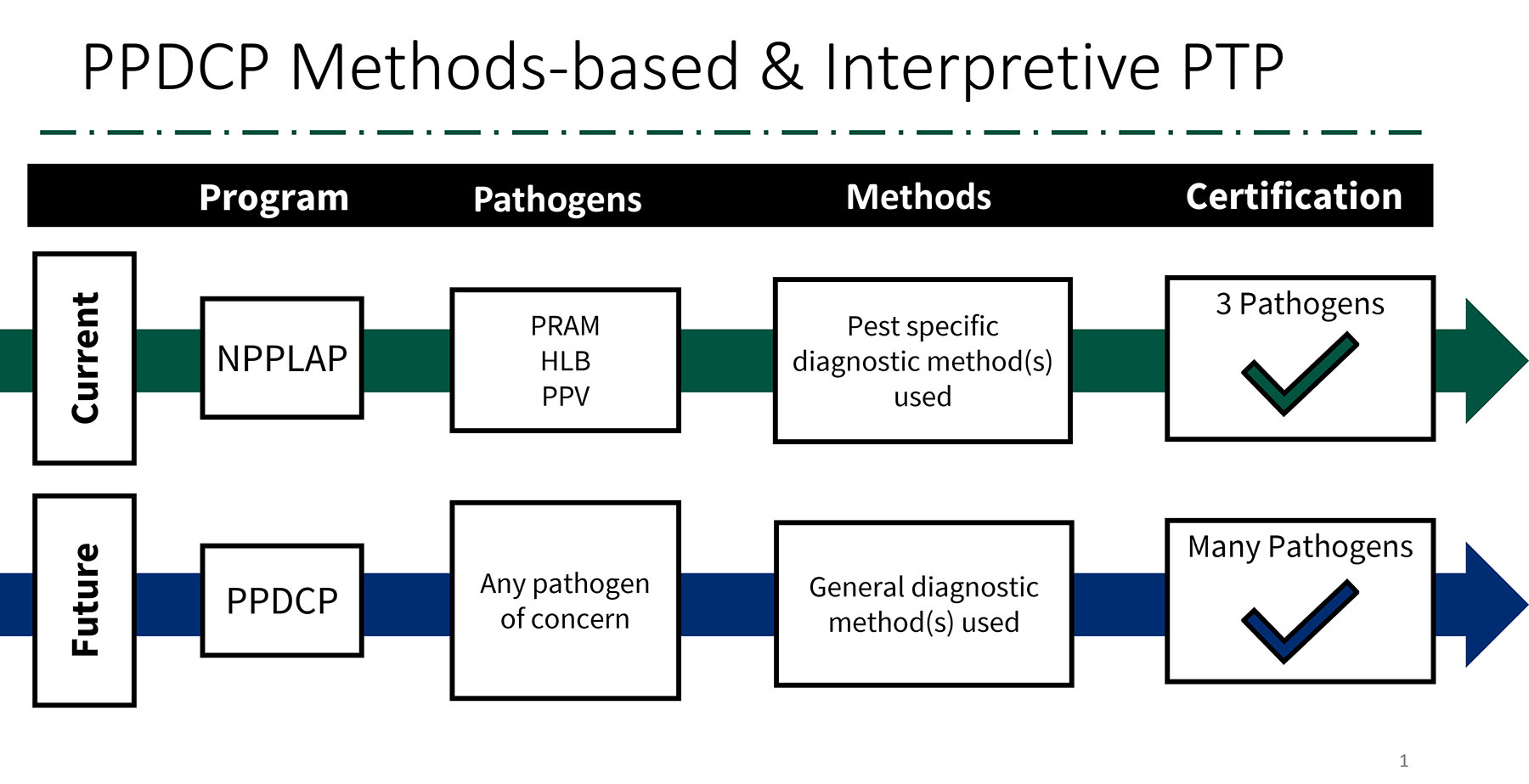
While uniform lab standards will be set up to participate in the program, the program will focus on individual diagnostician certification. We envision one annual lab-based PT panel designed to certify diagnosticians in their methods of choice: ELISA, DNA/RNA extraction, and different types of PCR assays. For example, if you choose ELISA certification, it will cover all the ELISA-based diagnostics you perform for PPQ-regulated pathogens like PRAM, CGMMV, ToBRFV etc. The same goes for PCR and RT-PCR; your base methods will be covered for your desired certification. 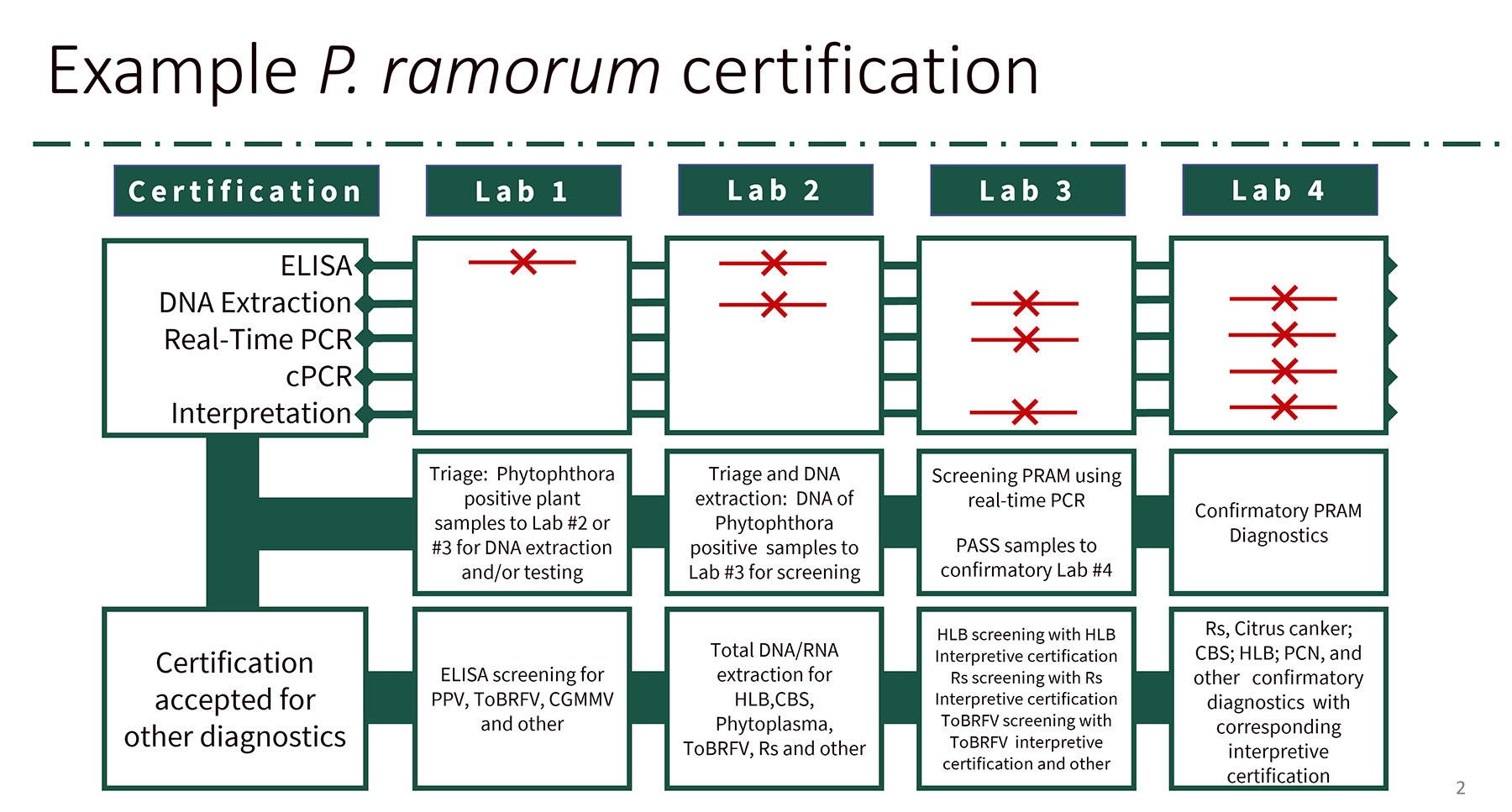
We all know that testing is only part of the diagnostic process. Results interpretation is just as important. To evaluate interpretation competency, we plan to produce pathogen-specific paper panels called “interpretive” proficiency tests. These panels will contain a set of results (gel photos, PCR traces, or results tables) to be evaluated and interpreted, as well as questions about Good Lab Practice (GLP), troubleshooting, and so on. With each interpretive panel you pass, you receive certification to interpret the diagnostic results of that test. We plan to offer interpretive panels annually for HLB, PRAM, CGMMV, and other pathogens. By combining the lab-based and interpretive panel certifications, labs can meet the requirements for providing diagnostics for multiple PPQ programs. The program also includes improvement in training and methods validation processes.
This program will be phased in over the next three years as to ensure minimal disruption to current survey activities. We introduced the program to PPQ, NPB, and NPDN management, and are working with all parties to tailor the program to maximize benefits and resources. We seek close collaboration with NPDN Committees and Dept of Ag laboratories to harmonize our efforts and draw upon our collective wealth of experience and resources. We want to better serve APHIS’ stakeholders, and most of all, you.
A few take-home messages:
- We are extending current HLB, PPV, and PRAM certifications until new proficiency test panels are developed and ready for distribution.
- Diagnostic work instructions will not change. You must still use pathogen-specific diagnostic work instructions for regulatory testing.
- More conversations and meetings will follow.
- We are working on setting up an e-mail address for PPDCP.
- Please get in touch with us if you have immediate certification needs or have questions.
For now, please send e-mails to me: vessela.a.mavrodieva@usda.gov.

